Aprea Therapeutics, Inc. (NASDAQ: APRE) and Karyopharm Therapeutics Inc. (NASDAQ: KPTI) are both clinical-stage biopharmaceutical companies specializing in oncology, each with unique therapeutic approaches and developmental pipelines. Below is a comparative analysis to assist investors in evaluating these two entities.
Company Overviews
Aprea Therapeutics, Inc. (APRE): Founded in 2002 and headquartered in Doylestown, Pennsylvania, Aprea focuses on precision oncology through synthetic lethality, developing novel cancer therapeutics targeting DNA damage response pathways. Its lead product candidate, ATRN-119, is a clinical-stage small molecule ATR inhibitor in development for solid tumor indications. Additionally, Aprea is developing ATRN-1051, an oral, small-molecule WEE1 inhibitor, which recently entered clinical trials.
Karyopharm Therapeutics Inc. (KPTI): Established in 2008 and based in Newton, Massachusetts, Karyopharm is dedicated to the discovery, development, and commercialization of novel first-in-class drugs directed against nuclear export for the treatment of cancer and other major diseases. Its lead product, XPOVIO® (selinexor), is an oral selective inhibitor of nuclear export (SINE) compound approved by the FDA for the treatment of multiple myeloma and diffuse large B-cell lymphoma.
Pipeline and Product Candidates
Aprea Therapeutics:
- ATRN-119: Currently in Phase I clinical trials for advanced solid tumors, ATRN-119 is designed to inhibit ATR, a key regulator of the DNA damage response, potentially enhancing the efficacy of cancer treatments.
- ATRN-1051: An oral WEE1 inhibitor that has recently entered clinical trials, targeting cell cycle regulation in cancer cells.
Karyopharm Therapeutics:
- XPOVIO® (selinexor): Approved for multiple indications, including relapsed or refractory multiple myeloma and diffuse large B-cell lymphoma.
- Eltanexor: A second-generation SINE compound in clinical development for myelodysplastic syndromes and other cancers.
- Verdinexor: In development for the treatment of viral infections and as a potential therapy for COVID-19.
Financial Performance
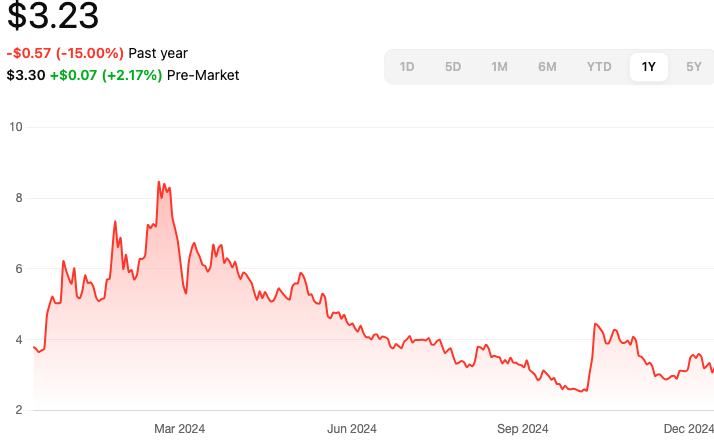
Aprea Therapeutics Inc (APRE)
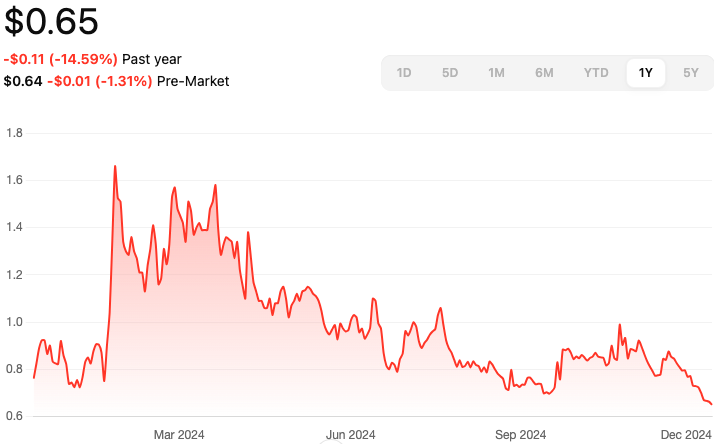
Karyopharm Therapeutics Inc (KPTI)
Recent Developments
Aprea Therapeutics:
- Clinical Advances: Recent reports indicate promising clinical advances with ATRN-119, leading to a reiterated Buy rating from analysts with a price target of $20.00.
- Financial Position: As of March 2024, Aprea reported $21.6 million in cash and cash equivalents, with a private placement financing in March 2024 raising upfront gross proceeds of approximately $16 million.
Karyopharm Therapeutics:
- Regulatory Approvals: Continued expansion of XPOVIO® indications, with recent FDA approvals for additional cancer treatments.
- Financial Position: As of the latest quarterly report, Karyopharm reported revenues primarily driven by XPOVIO® sales, with ongoing investments in research and development for pipeline expansion.
Conclusion
Aprea Therapeutics is focused on developing novel cancer therapeutics targeting DNA damage response pathways, with its lead candidates in early clinical stages. The company has a solid financial position to support ongoing research and development.
Karyopharm Therapeutics has successfully commercialized its lead product, XPOVIO®, generating revenue and supporting further pipeline development. The company continues to expand its product indications and invest in new therapeutic areas.
Investors should consider the clinical stage and financial health of Aprea Therapeutics, as well as the commercial success and pipeline expansion of Karyopharm Therapeutics, in line with their investment strategies and risk tolerance.
Marc has been involved in the Stock Market Media Industry for the last +5 years. After obtaining a college degree in engineering in France, he moved to Canada, where he created Money,eh?, a personal finance website.

