What Happened
Doseology (CSE: MOOD | OTCQB: DOSEF | FSE: VU70), recently announced that Doseology USA Inc., its wholly owned subsidiary operating in the United States, has entered into a confidential manufacturing agreement with a North America production partner after an extensive due diligence process. The due diligence involved operational review and assessment of compliance across multiple facilities including on site visits.
The purpose of the manufacturing agreement is to provide Doseology with commercially viable manufacturing capabilities for its oral stimulant pouch products, moving from product development to production readiness using external manufacturing capabilities.
Why This Matters
Obtaining a manufacturing partnership represents a major step in Doseology’s progression from product development to potentially commercializing those products. The selected partner has a manufacturing facility that is registered with the Food & Drug Administration (“FDA”) and certified under Good Manufacturing Practices (“GMP”) and ISO 9001:2015; therefore, it can be used to manufacture Doseology’s formulations and provide additional services including pouch filling, packaging, and logistics.
A manufacturing agreement with a compliant third party is essential for increasing product output beyond what can be achieved internally through research & development. Although the selection of a manufacturing partner does not mean that Doseology’s products will ultimately be successful commercially, the agreement provides Doseology with a working framework for producing its products that did not exist prior to the agreement and reduces some of the friction associated with establishing a manufacturing operation for new products that includes meeting production standards, quality control, and regulatory requirements.

What Doseology Does
Doseology (CSE: MOOD | OTCQB: DOSEF | FSE: VU70) is a company that produces consumer wellness and functional products utilizing a variety of technologies to produce precision-dosed oral stimulants and supplements. Doseology’s primary product line consists of oral stimulant pouches designed to deliver pre-measured quantities of active ingredients without burning, vaping, or consuming a liquid energy drink.
Some key aspects of Doseology’s products and position in the marketplace include:
- Consistent dosing and delivery: The typical amount of active ingredient in each Doseology pouch is in the range of tens of milligrams. Therefore, customers know exactly what they are receiving in each dose versus energy drinks that typically have between 150 and 300 milligrams of caffeine per serving.
- An alternative energy format: Pouch-based technology allows customers to consume smoke-free, sugar-free, and portable stimulants without having to consume liquids.
- A large addressable market: As Doseology operates at the intersection of the global energy supplement and nicotine-free pouch markets, it has a significant opportunity to capture a portion of the multi-billion dollar global functional stimulant market which is influenced by consumer trends away from combustible products and towards cleaner and less conspicuous energy formats.
In general, Doseology focuses on product format innovation, controlled dosing, and manufacturing compliance and less on rapid growth and expanding its brand.
Operational Considerations and Outlook for Execution
Although the terms of the manufacturing agreement remain confidential, its strategic importance lies in laying the groundwork for potential commercialization, rather than in generating immediate revenue. Doseology’s focus on creating operational readiness and compliance for potential future production ensures that any products produced in the future will meet regulatory and quality standards applicable in North America.
By selecting a manufacturing partner that has an FDA registered, GMP certified manufacturing facility, Doseology is positioned to operate in accordance with existing regulatory frameworks from day one, thereby reducing the potential for friction when scaling up production and when negotiating with distributors and retailers who need documentation to demonstrate quality and compliance controls.
Additionally, third-party manufacturing creates flexibility for Doseology, because instead of expending capital to create and validate its own facilities, it can vary production levels based on changes in market conditions. The modular nature of third-party manufacturing supports disciplined execution while allowing Doseology to preserve optionality relative to evolving product formats, changing demand signals, and regulatory pathways.
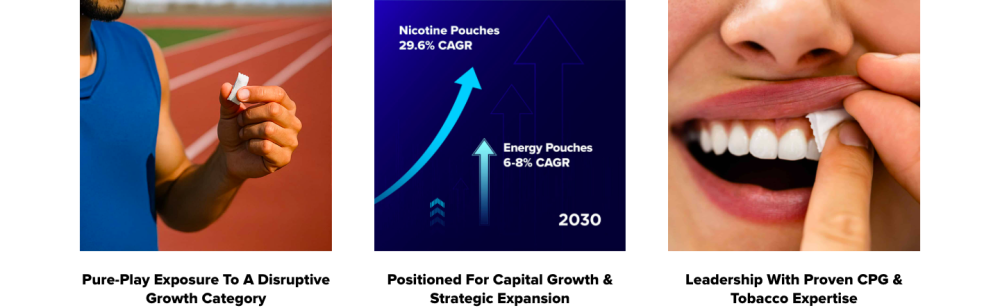
Wider Context and Business Position
The manufacturing agreement represents a component of a larger commercialization strategy for Doseology, and not a singular tipping point. Doseology remains committed to executing a phased strategy for preparing its products for commercialization, including developing its products, preparing them for regulatory approval, and creating the necessary operational infrastructure.
As Doseology begins to implement the next several steps in its commercialization roadmap, such as pilot runs, packaging validation, shelf life testing and negotiations with potential channel partners, it will continue to develop the operational capabilities that were missing in the past.
Over the long term, Doseology’s focus on manufacturing in North America could also help to position its brands around quality, traceability and compliance, all of which are important in consumer wellness and functional product categories, especially as scrutiny regarding sourcing and standards increases.
What to Watch for Going Forward
- Disclosure about the economic and/or timing of the manufacturing arrangement.
- Evidence of pilot production runs, batch validations, and/or third-party quality audit results.
- Updates related to the regulation of product classifications or commercialization pathways.
- Early signs of distribution-related discussions or commercial partnership activity.
Conclusion
Doseology (CSE: MOOD | OTCQB: DOSEF | FSE: VU70)’s manufacturing agreement demonstrates a methodical and intentional step toward operational maturity, rather than a near-term catalyst. By choosing to conduct due diligence, ensure compliance and create infrastructure prior to pursuing scale, Doseology is creating a foundation that should support future commercialization activities. Ultimately, the path to achieving sustained operational and commercial progress depends on Doseology’s ability to successfully execute on upcoming activities and translate the groundwork laid out in the manufacturing agreement into ongoing operational and commercial progress.
Marc has been involved in the Stock Market Media Industry for the last +5 years. After obtaining a college degree in engineering in France, he moved to Canada, where he created Money,eh?, a personal finance website.

