
NurExone Biologic Inc. is a TSXV-listed pharmaceutical company that is developing a platform for biologically-guided ExoTherapy to be delivered, non-invasively, to patients who suffer traumatic spinal cord injuries.
After securing the Orphan Drug Designation for ExoPTEN in the United States, NurExone has been granted Orphan Drug Designation for ExoPTEN in Europe as well.
Dr. Ina Sarel, Head of CMC, Quality, and Regulation at Nurexone Biologic, expressed enthusiasm about the development, stating, “Embarking on the European Orphan Drug Designation process marks a crucial milestone in our mission to bring life-changing treatments to patients in need around the world.”
TORONTO and HAIFA, Israel, (TSXV: NRX) (FSE: J90) (NRX.V) (the “Company” or “NurExone”), a biopharmaceutical company developing biologically-guided exosome therapy for patients with traumatic spinal cord injuries.
The FDA Orphan Drug Designation, while an exceptional win for the Company, has limitations. The same designation from the European Medicines Agency (EMA) for its groundbreaking ExoPTEN product gives NurExone global reach.
Orphan Drug Designation is granted to therapies addressing rare diseases, providing incentives to encourage the development of treatments for conditions affecting a small number of patients. Notable benefits of Orphan Drug Designation in Europe include ten years of market exclusivity in the European Union, fee reduction, financial incentives, and extended market protection.
Why is NurExone a Big Deal?
“Orphan-drug designation is expected to streamline our go-to-market, shorten our regulatory process, save the Company millions of dollars, and provide valuable market exclusivity. We appreciate the formal recognition of the potential impact of our therapy on the lives of patients suffering from acute spinal cord injuries,” said Dr. Shaltiel, CEO of NurExone Biologic, Ltd.
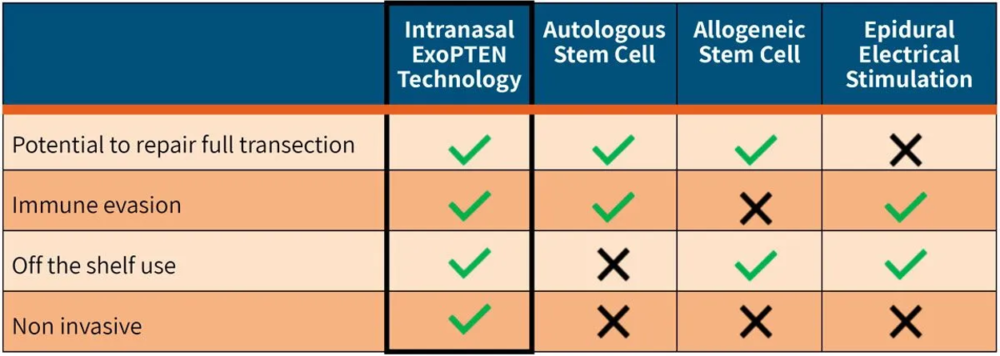
Initial indications from a pre-clinical study have demonstrated the potential for an off-the-shelf therapy for non-invasive administration shortly after spinal cord trauma. The product, which would not require personalization, is expected to reduce damage from a spinal cord injury and to improve the chance of functional recovery.
Bottom Line
NRX, at the moment, has a low but growing daily volume. Interestingly, the share quadrupled in the last 52-week period and is currently about 20% from the peak.
For investors, NRX is a great medical story, particularly given the increased prevalence of traumatic spine injuries. With the increase in significant conflicts, the need for this type of biological therapy is not only paramount, but they are critical to get to those folks suffering.
Please take a look, and I think you’ll be impressed.
Posted on Behalf of NurExone Biologic
